Purpose and objectives of the lesson:
– systematize students’ knowledge about the structure of the benzene molecule and methods for its preparation;
– form an understanding of the physical and chemical properties of benzene, teach how to compose equations for chemical reactions characteristic of benzene;
– continue to develop students’ skills in working with video materials and multimedia presentations.
Forms of work: frontal, individual.
Equipment: computer, multimedia projector, “Benzol” tables
Lesson progress
I. Organizational moment.
Teacher: Topic, goals, and objectives of the lesson.
II. Activation of students' knowledge.
- Frontal survey
- Aromatic hydrocarbons – ARENES
- Define aromatic hydrocarbons.
- Why are they called aromatic?
- A typical representative of aromatic hydrocarbons is...?
- Whose names are associated with the origin of benzene?
- What is the molecular formula of benzene?
- How many structural formulas of benzene?
- Type of hybridization?
- What bonds are there in a benzene molecule and how many?
- What are the most important sources of aromatic hydrocarbons?
- Other methods of obtaining?
- Name the benzene hamlogues.
- The structure of the benzene molecule (student message). (Slide 4).
- Independent work of students (for 5-7 minutes). (Slide 5).
- fill in the blanks in the definition of aromatic hydrocarbons;
- write the formulas of the given substances;
- complete the reduction of reactions producing aromatic hydrocarbons.
III. Learning new material.
1. Physical properties of benzene. (Slide 6).
Benzene is a colorless, volatile, flammable liquid with an unpleasant odor. It is lighter than water (=0.88 g/cm3) and does not mix with it, but it is soluble in organic solvents, and itself dissolves many substances well. Benzene boils at 80.1 C; when cooled, it easily solidifies into a white crystalline mass. Benzene and its vapors are poisonous. Systematic inhalation of its vapors causes anemia and leukemia.
– Video material (physical properties of benzene).
2. Chemical properties of benzene.
1) The chemical properties of benzene are determined by the structure of its molecule.
2) The aromatic system has increased stability.
3) Therefore, although benzene is an unsaturated hydrocarbon, it exhibits properties characteristic of saturated hydrocarbons (propensity for substitution reactions, resistance to oxidizing agents).
Substitution reactions.

Addition reactions (Slide 9).
Under certain conditions, benzene can also undergo addition reactions. In these reactions, the aromatic system is destroyed, so harsh conditions are required for them to occur.

Oxidation reactions. (Slide 10).
a) the ratio of benzene to bromine water and potassium permanganate (video material)
b) combustion of benzene
2C 6 H 6 + 15O 2 –> 2CO 2 + 6H 2 O
IV. Consolidation.
(Slide 11).- Benzene reacts with each substance in the set:
a) Br 2, O 2, KMnO 4
b) H 2 O, HNO 3, CI 2
c) CI 2, O 2, HNO 3
d) HCI, Br 2, H 2
Write the equations for the reactions of benzene with the substances of this set, indicate the conditions for their occurrence.
V. Homework.
Identify the substances X, Y, Z in the transformation scheme:
Literature:
- Rudzitis G.E., Feldman F.G. Organic chemistry: Textbook for 10 grades of general education institutions. – 8th ed. – M.: Education, 2002.
- Novoshinsky I.I., Novoshinskaya N.S. Organic chemistry. 11th grade: Textbook for general education institutions. – M.: Publishing house “Education”, 2005.
Mrs. Khimiya finally and irrevocably acquired such a compound as benzene only in 1833. Benzene is a compound that has a hot-tempered, one might even say explosive, character. How did you find out?
Story
Johann Glauber in 1649 turned his attention to a compound that was successfully formed when a chemist was processing coal tar. But it wished to remain incognito.
About 170 years later, or to be much more precise, in the mid-twenties of the 19th century, by chance, benzene was extracted from the illuminating gas, namely from the released condensate. Humanity owes such efforts to Michael Faraday, a scientist from England.
The baton for the acquisition of benzene was taken over by the German Eilgard Mitscherlich. This happened during the processing of anhydrous calcium salts of benzoic acid. Perhaps that is why the compound was given such a name - benzene. Alternatively, the scientist called it gasoline. Incense, if translated from Arabic.
Benzene burns beautifully and brightly; in connection with these observations, Auguste Laurent recommended calling it “fen” or “benzene”. Bright, shining - if translated from Greek.
Based on the concept of the nature of electronic communication and the qualities of benzene, the scientist provided the molecule of the compound in the form of the following image. This is a hexagon. A circle is inscribed in it. The above suggests that benzene has a complete electron cloud, which safely encloses six (without exception) carbon atoms of the cycle. No fastened binary bonds are observed.
Benzene was previously used as a solvent. But basically, as they say, he was not a member, did not participate, was not involved. But this is in the 19th century. Significant changes took place in the 20th century. The properties of benzene express the most valuable qualities that have helped it become more popular. The octane number, which turned out to be high, made it possible to use it as a fuel element for refueling cars. This action was the impetus for the extensive withdrawal of benzene, its extraction is carried out as a secondary product of coking steel production.
By the forties, benzene began to be used in the chemical field in the manufacture of substances that quickly explode. The 20th century crowned itself with the fact that the oil refining industry produced so much benzene that it began to supply the chemical industry.
Characteristics of benzene
Unsaturated hydrocarbons are very similar to benzene. For example, the ethylene hydrocarbon series characterizes itself as an unsaturated hydrocarbon. It is characterized by an addition reaction. Benzene readily enters into all this thanks to the atoms that are in the same plane. And as a fact - a conjugate electron cloud.

If a benzene ring is present in the formula, then we can come to the elementary conclusion that it is benzene, the structural formula of which looks exactly like this.
Physical properties
Benzene is a liquid that has no color, but has a regrettable odor. Benzene melts when the temperature reaches 5.52 degrees Celsius. Boils at 80.1. The density is 0.879 g/cm 3, the molar mass is 78.11 g/mol. When burning it smokes a lot. Forms explosive compounds when air enters. rocks (gasoline, ether and others) combine with the described substance without problems. Creates an azeotropic compound with water. Heating before vaporization begins at 69.25 degrees (91% benzene). At 25 degrees Celsius it can dissolve in water 1.79 g/l.
Chemical properties
Benzene reacts with sulfuric and nitric acid. And also with alkenes, halogens, chloroalkanes. The substitution reaction is what is characteristic of it. The pressure temperature affects the breakthrough of the benzene ring, which occurs under rather harsh conditions.
We can consider each benzene reaction equation in more detail.
1. Electrophilic substitution. Bromine, in the presence of a catalyst, reacts with chlorine. As a result, we obtain chlorobenzene:
С6H6+3Cl2 → C6H5Cl + HCl
2. Friedel-Crafts reaction, or alkylation of benzene. The appearance of alkylbenzenes occurs due to the combination with alkanes, which are halogen derivatives:
C6H6 + C2H5Br → C6H5C2H5 + HBr
3. Electrophilic substitution. Here the reaction of nitration and sulfonation takes place. The equation for benzene will look like this:
C6H6 + H2SO4 → C6H5SO3H + H2O
C6H6 + HNO3 → C6H5NO2 + H2O
4. Benzene when burning:
2C6H6 + 15O2 → 12CO2 + 6H2O
Under certain conditions, it exhibits a character characteristic of saturated hydrocarbons. The P-electron cloud, which is located in the structure of the substance in question, explains these reactions.
Different types of benzene depend on special technology. This is where petroleum benzene is labeled. For example, purified and highly purified, for synthesis. I would like to separately note benzene homologues, and more specifically, their chemical properties. These are alkylbenzenes.

Benzene homologues react much more readily. But the above reactions of benzene, namely homologues, take place with some differences.
Halogenation of alkylbenzenes
The form of the equation is as follows:
C6H5-CH3 + Br = C6H5-CH2Br + HBr.
The tendency of bromine into the benzene ring is not observed. It comes out into the chain from the side. But thanks to the Al(+3) salt catalyst, bromine easily enters the ring.
Nitration of alkylbenzenes
Thanks to sulfuric and nitric acids, benzenes and alkylbenzenes are nitrated. Reactive alkylbenzenes. Two of the presented three products are obtained - these are para- and ortho-isomers. You can write one of the formulas:
C6H5 - CH3 + 3HNO3 → C6H2CH3 (NO2)3.
Oxidation
This is unacceptable for benzene. But alkylbenzenes react readily. For example, benzoic acid. The formula is given below:
C6H5CH3 + [O] → C6H5COOH.
Alkylbenzene and benzene, their hydrogenation
In the presence of an amplifier, hydrogen begins to react with benzene, resulting in the formation of cyclohexane, as discussed above. Likewise, alkylbenzenes are easily converted to alkylcyclohexanes. To obtain alkylcyclohexane, it is necessary to hydrogenate the desired alkylbenzene. This is basically a necessary procedure to produce a pure product. And this is not all the reactions of benzene and alkylbenzene.
Benzene production. Industry
The foundation of such production is based on the processing of components: toluene, naphtha, tar, which is released during cracking of coal, and others. Therefore, benzene is produced at petrochemical and metallurgical enterprises. It is important to know how to obtain benzene of varying degrees of purity, because the manufacturing principle and purpose directly depend on the brand of this substance.
The lion's share is produced by thermocatalytic reforming of the caustobiolite part, boiling at 65 degrees, having an extract effect, distillation with dimethylformamide.
When producing ethylene and propylene, liquid products are obtained that are formed during the decomposition of inorganic and organic compounds under the influence of heat. Benzene is isolated from them. But, unfortunately, there is not so much source material for this option for benzene extraction. Therefore, the substance we are interested in is extracted by reforming. By this method the volume of benzene is increased.

By dealkylation at a temperature of 610-830 degrees with a plus sign, in the presence of steam formed by the boiling of water and hydrogen, benzene is obtained from toluene. There is another option - catalytic. When the presence of zeolites, or, alternatively, oxide catalysts, is observed, subject to a temperature regime of 227-627 degrees.
There is another, older, method for developing benzene. Using absorption by absorbents of organic origin, it is isolated from the final result of coking coal. The product is a vapor-gas product and has been pre-cooled. For example, oil is used, the source of which is petroleum or coal. When distillation is carried out with steam, the absorbent is separated. Hydrotreating helps remove excess substances from crude benzene.
Coal raw materials
In metallurgy, when using coal, or, to be more precise, dry distilling it, coke is obtained. During this procedure, the air supply is limited. Do not forget that coal is heated to a temperature of 1200-1500 Celsius.
Coal chemical benzene needs thorough purification. It is imperative to get rid of methyl cyclohexane and its friend n-heptane. should also be confiscated. Benzene faces a process of separation and purification, which will be carried out more than once.
The method described above is the oldest, but over time it loses its high position.
Oil fractions
0.3-1.2% - these are the composition indicators of our hero in crude oil. Meager indicators to invest money and effort. It is best to use an industrial procedure for processing petroleum fractions. That is, catalytic reforming. In the presence of an aluminum-platinum-rhenium amplifier, the percentage of aromatic carbohydrates increases, and the indicator that determines the ability of the fuel not to spontaneously ignite during its compression increases.
Pyrolysis resins
If we extract our petroleum product from non-solid raw materials, namely by pyrolysis of propylene and ethylene arising during the production, then this approach will be the most acceptable. To be precise, benzene is released from the pyrocondensate. The decomposition of certain proportions requires hydrotreating. During cleaning, sulfur and unsaturated mixtures are removed. The initial result contained xylene, toluene, and benzene. Using distillation, which is extractive, the BTK group is separated to produce benzene.

Hydrodealkylation of toluene
The main characters of the process, a cocktail of hydrogen flow and toluene, are fed heated into the reactor. Toluene passes through the catalyst bed. During this process, the methyl group is separated to form benzene. A certain method of cleansing is appropriate here. The result is a highly pure substance (for nitration).
Disproportionation of toluene
As a result of the rejection of the methyl class, creation occurs to benzene, and xylene is oxidized. Transalkylation has been observed in this process. The catalytic effect occurs thanks to palladium, platinum and neodymium, which are located on aluminum oxide.
Taluene and hydrogen are supplied to the reactor with a stable catalyst bed. Its purpose is to keep hydrocarbons from settling on the catalyst plane. The stream that leaves the reactor is cooled, and hydrogen is safely recovered for recycling. What's left is distilled three times. At the initial stage, compounds that are non-aromatic are removed. Benzene is extracted second, and the last step is the separation of xylenes.
Acetylene trimerization
Thanks to the work of the French physical chemist Marcelin Berthelot, benzene began to be produced from acetylene. But what stood out was a heavy cocktail of many other elements. The question was how to lower the reaction temperature. The answer was received only in the late forties of the 20th century. V. Reppe found the appropriate catalyst, it turned out to be nickel. Trimerization is the only option to obtain benzene from acetylene.

Benzene is formed using activated carbon. At high heat levels, acetylene passes over the coal. Benzene is released if the temperature is at least 410 degrees. At the same time, various aromatic hydrocarbons are also born. Therefore, you need good equipment that can efficiently clean acetylene. With such a labor-intensive method as trimerization, a lot of acetylene is consumed. To obtain 15 ml of benzene, take 20 liters of acetylene. You can see how it looks and the reaction will not take long.
3C2H2 → C6H6 (Zelinsky equation).
3CH → CH = (t, kat) = C6H6.
Where is benzene used?
Benzene is a fairly popular brainchild of chemistry. It was especially often noticed how benzene was used in the production of cumene, cyclohexane, and ethylbenzene. To create styrene, you cannot do without ethylbenzene. The starting material for the production of caprolactam is cyclohexane. When making thermoplastic resin, caprolactam is used. The described substance is indispensable in the manufacture of various paints and varnishes.
How dangerous is benzene?
Benzene is a toxic substance. The manifestation of a feeling of malaise, which is accompanied by nausea and severe dizziness, is a sign of poisoning. Even death cannot be ruled out. A feeling of indescribable delight is no less alarming bells for benzene poisoning.
Benzene in liquid form causes skin irritation. Benzene vapors easily penetrate even intact skin. With very short-term contacts with the substance in a small dose, but on a regular basis, unpleasant consequences will not be long in coming. This may be bone marrow damage and acute leukemia of various types.

In addition, the substance causes addiction in humans. Benzene acts like dope. Tobacco smoke produces a tar-like product. When they studied it, they came to the conclusion that its contents were unsafe for humans. In addition to the presence of nicotine, the presence of aromatic carbohydrates such as benzopyrene was also discovered. A distinctive feature of benzopyrene is that it is carcinogenic. They have a very harmful effect. For example, they cause cancer.
Despite the above, benzene is a starting raw material for the production of a variety of medicines, plastics, synthetic rubber and, of course, dyes. This is the most common brainchild of chemistry and an aromatic compound.
The cyclic structure of benzene was first proposed by F.A. Kekule in 1865

Friedrich August Kekule von Stradonitz - an outstanding German chemist of the 19th century. In 1854, he discovered the first organic compound containing sulfur - thioacetic acid (thioethanoic acid). In addition, he established the structure of diazo compounds. However, his most famous contribution to the development of chemistry is the establishment of the structure of benzene (1866). Kekulé showed that the double bonds of benzene alternated around the ring (an idea that first occurred to him in a dream). He later showed that the two possible double bond arrangements are identical and that the benzene ring is a hybrid between these two structures. Thus, he anticipated the idea of resonance (mesomerism), which appeared in the theory of chemical bonding in the early 1930s.
If benzene really had such a structure, then its 1,2-disubstituted derivatives should have two isomers. For example,

However, none of the 1,2-disubstituted benzenes can be isolated into two isomers.
Therefore, Kekule subsequently suggested that the benzene molecule exists as two structures that quickly transform into each other:

Note that such schematic representations of benzene molecules and their derivatives usually do not indicate the hydrogen atoms attached to the carbon atoms of the benzene ring.
In modern chemistry, the benzene molecule is considered as a resonant hybrid of these two limiting resonant forms (see Section 2.1). Another description of the benzene molecule is based on a consideration of its molecular orbitals. In Sect. 3.1 it was indicated that -electrons located in -bonding orbitals are delocalized between all carbon atoms of the benzene ring and form an -electron cloud. In accordance with this representation, the benzene molecule can be conventionally depicted as follows:
Experimental data confirm the presence of just such a structure in benzene. If benzene had the structure that Kekulé originally proposed, with three conjugated double bonds, then benzene should undergo addition reactions like alkenes. However, as mentioned above, benzene does not undergo addition reactions. In addition, benzene is more stable than if it had three isolated double bonds. In Sect. 5.3 it was indicated that the enthalpy of benzene hydrogenation to form cyclohexane has a greater negative
Table 18.3. Length of various carbon-carbon bonds


Rice. 18.6. Geometric structure of the benzene molecule.
value than triple the enthalpy of hydrogenation of cyclohexene. The difference between these quantities is usually called the enthalpy of delocalization, resonance energy or stabilization energy of benzene.
All carbon-carbon bonds in the benzene ring have the same length, which is shorter than the length of the C-C bonds in alkanes, but longer than the length of the C=C bonds in alkenes (Table 18.3). This confirms that the carbon-carbon bonds in benzene are a hybrid between single and double bonds.
The benzene molecule has a flat structure, which is shown in Fig. 18.6.
Physical properties
Benzene under normal conditions is a colorless liquid that freezes at 5.5 °C and boils at 80 °C. It has a characteristic pleasant odor, but, as mentioned above, is highly toxic. Benzene does not mix with water and in a benzene system, water forms the upper of the two layers. However, it is soluble in non-polar organic solvents and is itself a good solvent for other organic compounds.
Chemical properties
Although benzene undergoes certain addition reactions (see below), it does not exhibit the reactivity typical of alkenes. For example, it does not discolor bromine water or -ion solution. Moreover, benzene is not
enters into addition reactions with strong acids, such as hydrochloric or sulfuric acid.
At the same time, benzene takes part in a number of electrophilic substitution reactions. The products of this type of reaction are aromatic compounds, since in these reactions the delocalized -electronic system of benzene is retained. The general mechanism for replacing a hydrogen atom on the benzene ring with an electrophile is described in Section. 17.3. Examples of electrophilic substitution of benzene are its nitration, halogenation, sulfonation and Friedel-Crafts reactions.
Nitration. Benzene can be nitrated (a group added to it) by treating it with a mixture of concentrated nitric and sulfuric acids:

Nitrobenzene
The conditions for this reaction and its mechanism are described in section. 17.3.
Nitrobenzene is a pale yellow liquid with a characteristic almond odor. When benzene is nitrated, in addition to nitrobenzene, crystals of 1,3-dinitrobenzene are also formed, which is the product of the following reaction:

Halogenation. If you mix benzene with chlorine or bromine in the dark, no reaction will occur. However, in the presence of catalysts possessing the properties of Lewis acids, electrophilic substitution reactions occur in such mixtures. Typical catalysts for these reactions are iron(III) bromide and aluminum chloride. The action of these catalysts is that they create polarization in the halogen molecules, which then form a complex with the catalyst:
although there is no direct evidence that free ions are formed in this case. The mechanism of benzene bromination using iron (III) bromide as an ion carrier can be represented as follows:

Sulfonation. Benzene can be sulfonated (replace a hydrogen atom with a sulfo group) by refluxing its mixture with concentrated sulfuric acid for several hours. Instead, benzene can be carefully heated in a mixture with fuming sulfuric acid. Fuming sulfuric acid contains sulfur trioxide. The mechanism of this reaction can be represented by the diagram

Friedel-Crafts reactions. Friedel-Crafts reactions were originally called condensation reactions between aromatic compounds and alkyl halides in the presence of an anhydrous aluminum chloride catalyst.
In condensation reactions, two molecules of reagents (or one reagent) combine with each other, forming a molecule of a new compound, while a molecule of some simple compound, such as water or hydrogen chloride, is split off (eliminates) from them.
Currently, the Friedel-Crafts reaction is called any electrophilic substitution of an aromatic compound in which the role of an electrophile is played by a carbocation or a highly polarized complex with a positively charged carbon atom. The electrophilic agent, as a rule, is an alkyl halide or chloride of some carboxylic acid, although it can also be, for example, an alkene or an alcohol. Anhydrous aluminum chloride is usually used as a catalyst for these reactions. Friedel-Crafts reactions are usually divided into two types: alkylation and acylation.
Alkylation. In this type of Friedel-Crafts reaction, one or more hydrogen atoms on the benzene ring are replaced by alkyl groups. For example, when a mixture of benzene and chloromethane is gently heated in the presence of anhydrous aluminum chloride, methylbenzene is formed. Chloromethane plays the role of an electrophilic agent in this reaction. It is polarized by aluminum chloride in the same way as halogen molecules:
The mechanism of the reaction under consideration can be presented as follows:

It should be noted that in this condensation reaction between benzene and chloromethane, a hydrogen chloride molecule is eliminated. Note also that the real existence of the metal carbocation in the form of a free ion is doubtful.
Alkylation of benzene with chloromethane in the presence of a catalyst - anhydrous aluminum chloride does not result in the formation of methylbenzene. In this reaction, further alkylation of the benzene ring occurs, leading to the formation of 1,2-dimethylbenzene:

Acylation. In this type of Friedel-Crafts reaction, a hydrogen atom on the benzene ring is replaced by an acyl group, resulting in the formation of an aromatic ketone.
The acyl group has the general formula
The systematic name of an acyl compound is formed by replacing the suffix and ending -ova in the name of the corresponding carboxylic acid, of which this acyl compound is a derivative, with the suffix -(o) yl. For example

The acylation of benzene is carried out using the chloride or anhydride of any carboxylic acid in the presence of a catalyst, anhydrous aluminum chloride. For example

This reaction is a condensation in which a hydrogen chloride molecule is eliminated. Note also that the name "phenyl" is often used to refer to the benzene ring in compounds where benzene is not the main group:

Addition reactions. Although benzene is most characterized by electrophilic substitution reactions, it also undergoes some addition reactions. We have already met one of them. We are talking about the hydrogenation of benzene (see section 5.3). When a mixture of benzene and hydrogen is passed over the surface of a finely ground nickel catalyst at a temperature of 150-160 °C, a whole sequence of reactions occurs, which ends with the formation of cyclohexane. The overall stoichiometric equation for this reaction can be represented as follows:

When exposed to ultraviolet radiation or direct sunlight, benzene also reacts with chlorine. This reaction occurs via a complex radical mechanism. Its final product is 1,2,3,4,5,6-hexachlorocyclohexane:

A similar reaction occurs between benzene and bromine under the influence of ultraviolet radiation or sunlight.
Oxidation. Benzene and the benzene ring in other aromatic compounds are, generally speaking, resistant to oxidation even by such strong oxidizing agents as an acidic or alkaline solution of potassium permanganate. However, benzene and other aromatic compounds burn in air or oxygen to produce a very smoky flame, which is typical of hydrocarbons with a high relative carbon content.

Benzene homologs are capable of reacting in two directions with the participation of an aromatic core and a side chain (alkyl groups), depending on the nature of the reagent.
1.Reactions on the aromatic ring
Due to the donor effect of the alkyl group, the S E ArH reactions proceed in ortho- And pair-position of the aromatic ring, while the conditions are milder than for benzene.
a) halogenation

b) nitration


Notice how, as the number of acceptor groups (-NO2) increases, the temperature of the nitration reactions increases.
c) sulfonation

The reaction predominantly produces n-isomer.
d) alkylation

e) acylation

2. Side chain reactions
The alkyl fragment of the benzene molecule enters into S R reactions involving the carbon atom in α -position (benzylic position).


Oxidation of all benzene homologues with KMnO 4 /100°C leads to the formation of benzoic acid.
Condensed arenas

Condensed arenes are aromatic systems (n=2 and 3). The degree of aromaticity of condensed arenes is lower than for benzene. They are characterized by electrophilic substitution reactions, addition reactions and oxidation reactions, which occur under milder conditions than for benzene.
Reactivity of naphthalene
The S E ArH reactions for naphthalene proceed mainly according to α -position, with the exception of sulfonation. The electrophilic addition of Ad E occurs at positions 1,4, with naphthalene exhibiting the properties of conjugated dienes.
1. Electrophilic substitution reactions,S E ArH


2.Reactions of electrophilic addition, reduction and oxidation.


Reactivity of anthracene and phenanthrene
The reactions of electrophilic substitution, S E ArH and electrophilic addition, Ad E for anthracene occur predominantly at positions 9 and 10 (see the diagram below).

The reactions of electrophilic substitution, S E ArH and electrophilic addition, Ad E for phenanthrene occur predominantly at positions 9 and 10, as for anthracene (see the diagram below).

Oxidation and reduction reactions for anthracene and phenanthrene.


Structures of some drugs based on naphthalene, anthracene and phenanthrene
Naphthyzin(naphazoline, sanorin)

vasoconstrictor effect(treatment of rhinitis, sinusitis)
(the original structure is emphasized in the title, pay attention to the numbering)
Naftifin

antifungal action (treatment of dermatitis)
Nabumethon

anti-inflammatory, antipyretic, analgesic effect(treatment of osteoarthritis, rheumatoid arthritis).
Nadolol

(the term cis, in this case, denotes the relative position of hydroxyl groups)
hypotensive(lowers blood pressure) and antiarrhythmic effect
Morphine, codeine

Test questions for the chapter “ARENAS”
1. What properties of benzene distinguish it from other unsaturated compounds - alkenes, alkynes? What does the term “aromatic compound” mean?
2. Write the structural formulas of the compounds: a) ethylbenzene; b) 1,3-dimethylbenzene ( m -xylene); c) 1,3,5-trimethylbenzene (mesitylene); d) isopropylbenzene (cumene); e) 3-phenylpentane; f) vinylbenzene (styrene); g) phenylacetylene; h) trance -diphenylethylene ( trance -stilbene).
3. Characterize the structural features of compounds exhibiting aromaticity. Formulate Hückel's rule. Which of the following compounds are aromatic:

4. Compare the ratio of cyclohexene and benzene to the following reagents under the indicated conditions : a) Br 2 (H 2 O, 20 C); b) KMnO 4 (H 2 O.0 C); c) N 2 SO 4 (conc.), 20 C; d) H 2 (Pd, 30 C); d) O 3 , then H 2 O(Zn); f) HBr.
5. Write the structural formulas of monosubstituted benzenes formed in the reactions of benzene with the following reagents: a) N 2 SO 4 (conc.); b) HNO 3 ; N 2 SO 4 (conc.); c) Br 2 /Fe; d) Cl 2 /AlCl 3 ; e) CH 3 Br/AlBr 3 ; e) CH 3 COCl/AlСl 3 . Name the reactions and their products. Indicate with which electrophile benzene reacts in each specific case.
6. Give a general scheme for the interaction of benzene with an electrophilic reagent ( E + ). Name the intermediate complexes. Which step usually determines the rate of a reaction? Give a graph of the change in potential energy of the reaction under consideration.
7. Define the following concepts: a) transition state; b) intermediate connection; c) -complex; d)-complex. Which of them are identical? Illustrate these concepts using the example of benzene bromination in the presence of a catalyst. FeBr 3 .
8. Using the example of reactions of ethene and benzene with bromine, compare the mechanism of electrophilic addition in alkenes with the mechanism of electrophilic substitution in the aromatic series. At what stage is the difference observed and why?
9. Using inductive and mesomeric effects, describe the interaction of the substituent with the benzene ring in the indicated compounds:

Label the electron-donating (ED) and electron-withdrawing (EA) substituents.
10. Write schemes for mononitration of compounds: a) phenol; b) benzenesulfonic acids; c) isopropylbenzene; d) chlorobenzene. For which compound should the relative rate of substitution be the highest and why?
11. The formation of what products should be expected during monosulfonation of compounds: a) toluene; b) nitrobenzene; c) benzoic acid; d) bromobenzene? Which compound should be sulfonated the easiest? Why?
12. Arrange the following compounds in order of increasing reactivity when brominating them into a benzene ring: a) benzene; b) phenol; c) benzaldehyde; d) ethylbenzene. Give an explanation.
13. Name the following hydrocarbons:

14. Write the reactions of benzene with the following reagents : a) Cl 2 (Fe); b) 3Cl 2 (light); c) HNO 3 (H 2 SO 4 ); d) O 2 (air) (V 2 ABOUT 5 , 450 C); e) 3O 3 , then N 2 O(Zn); e) H 2 SO 4 (oleum); g) 3H 2 (Ni, 200 C,p ). What are the features of addition reactions in benzene?
15. Write the reactions of toluene with the indicated reagents : a) 3H 2 (Ni, 200 C, 9806.7 kPa); b) KMnO 4 V N 2 ABOUT; c*) Cl 2 , light; d*) Cl 2 (Fe); e*) CH 3 Cl(AlCl 3 ); e*) CH 3 COCl (AlCl 3 ); g) HNO 3 (H 2 SO 4 ). For reactions marked with an asterisk, give mechanisms.
16. Write the reactions of nitration of ethylbenzene under the indicated conditions: a) 65% HNO 3 +H 2 SO 4 (conc.); b) 10% HNO 3 , heating, pressure. Bring the mechanisms.
17. Compare the ratio of isopropylbenzene to bromine: a) in the presence AlBr 3 ; b) under lighting and heating. Give the reactions and their mechanisms.
18. What compounds are formed from ethylbenzene and n -xylene under the action of the indicated oxidizing agents: a) O 3 , then H 2 O(Zn); b) KMnO 4 in H 2 ABOUT,t ; c) K 2 Cr 2 O 7 in H 2 SO 4 , t ?
19. By means of what reactions can the following pairs of compounds be distinguished: a) ethylbenzene and m -xylene; b) ethylbenzene and styrene; c) styrene and phenylacetylene; G) O - And n -xylenes?
20. Which compounds are the products of the reactions below:

21. Based on benzene and any other reagents, obtain the compounds below: a) n -rubs -butyltoluene; b) ethyl- n -tolyl ketone; c) alylbenzene; G) n -bromobenzoic acid.
22. Name the main compounds formed in the following reactions:

The first group of reactions are substitution reactions. We said that arenes do not have multiple bonds in the structure of the molecule, but contain a conjugated system of six electrons, which is very stable and gives additional strength to the benzene ring. Therefore, in chemical reactions, the replacement of hydrogen atoms occurs first, and not the destruction of the benzene ring.
We have already encountered substitution reactions when talking about alkanes, but for them these reactions followed a radical mechanism, while arenes are characterized by an ionic mechanism of substitution reactions.
First chemical property halogenation. Replacement of a hydrogen atom with a halogen atom, chlorine or bromine.
The reaction occurs when heated and always with the participation of a catalyst. In the case of chlorine, it could be aluminum chloride or ferric chloride three. The catalyst polarizes the halogen molecule, causing heterolytic bond cleavage and producing ions.
Chlorine is a positively charged ion and reacts with benzene.
If the reaction occurs with bromine, then the catalyst is iron bromide or aluminum bromide.

It is important to note that the reaction occurs with molecular bromine and not with bromine water. Benzene does not react with bromine water.
The halogenation of benzene homologues has its own characteristics. In the toluene molecule, the methyl group facilitates substitution in the ring, reactivity increases, and the reaction occurs under milder conditions, that is, at room temperature.

It is important to note that substitution always occurs in the ortho and para positions, so a mixture of isomers is obtained.
Second property nitration of benzene, introduction of a nitro group into the benzene ring.

A heavy yellowish liquid with the smell of bitter almonds is formed nitrobenzene, so the reaction can be qualitative to benzene. For nitration, a nitrating mixture of concentrated nitric and sulfuric acids is used. The reaction is carried out by heating.
Let me remind you that for the nitration of alkanes in the Konovalov reaction, diluted nitric acid was used without the addition of sulfuric acid.
During the nitration of toluene, as well as during halogenation, a mixture of ortho- and para-isomers is formed.

Third property alkylation of benzene with haloalkanes.

This reaction allows the introduction of a hydrocarbon radical into the benzene ring and can be considered a method for producing benzene homologues. Aluminum chloride is used as a catalyst, which promotes the decomposition of the haloalkane molecule into ions. Heating is also necessary.
Fourth property alkylation of benzene with alkenes.
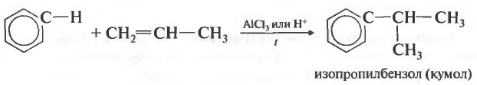
In this way you can obtain, for example, cumene or ethylbenzene. Catalyst aluminum chloride.
2. Addition reactions to benzene
The second group of reactions are addition reactions. We said that these reactions are not typical, but they are possible under fairly stringent conditions with the destruction of the pi-electron cloud and the formation of six sigma bonds.
Fifth property in the general list hydrogenation, addition of hydrogen.
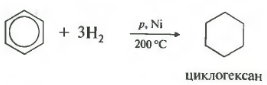
Temperature, pressure, catalyst nickel or platinum. Toluene can react in the same way.
Sixth property chlorination. Please note that we are talking specifically about interaction with chlorine, since bromine does not enter into this reaction.

The reaction occurs under harsh ultraviolet irradiation. Hexachlorocyclohexane, another name for hexachlorane, a solid, is formed.
It is important to remember that for benzene not possible addition reactions of hydrogen halides (hydrohalogenation) and addition of water (hydration).
3. Substitution in the side chain of benzene homologues
The third group of reactions concerns only benzene homologues - this is a substitution in the side chain.
Seventh property in the general list halogenation at the alpha carbon atom in the side chain.

The reaction occurs when heated or irradiated and always only at the alpha carbon. As halogenation continues, the second halogen atom will return to the alpha position.
4. Oxidation of benzene homologues
The fourth group of reactions is oxidation.
The benzene ring is too strong, so benzene does not oxidize potassium permanganate does not discolor its solution. This is very important to remember.
But benzene homologues are oxidized by an acidified solution of potassium permanganate when heated. And this is the eighth chemical property.
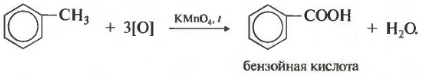
This produces benzoic acid. Discoloration of the solution is observed. In this case, no matter how long the carbon chain of the substituent is, it always breaks after the first carbon atom and the alpha atom is oxidized to a carboxyl group with the formation of benzoic acid. The remainder of the molecule is oxidized to the corresponding acid or, if it is only one carbon atom, to carbon dioxide.
If a benzene homologue has more than one hydrocarbon substituent on the aromatic ring, then oxidation occurs according to the same rules - the carbon located in the alpha position is oxidized.

This example produces a dibasic aromatic acid called phthalic acid.
I would especially like to note the oxidation of cumene, isopropylbenzene, by atmospheric oxygen in the presence of sulfuric acid.
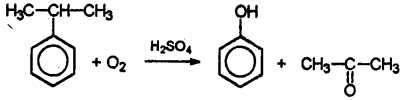
This is the so-called cumene method for producing phenol. As a rule, one encounters this reaction in matters related to the production of phenol. This is an industrial method.
Ninth property combustion, complete oxidation with oxygen. Benzene and its homologues burn to carbon dioxide and water.
Let us write the combustion equation of benzene in general form.
According to the law of conservation of mass, there should be as many atoms on the left as there are atoms on the right. Because in chemical reactions, atoms do not disappear, but the order of bonds between them simply changes. So there will be as many carbon dioxide molecules as there are carbon atoms in the arene molecule, since the molecule contains one carbon atom. That is, n CO 2 molecules. There will be two times fewer water molecules than hydrogen atoms, that is (2n-6)/2, which means n-3.
There are the same number of oxygen atoms on the left and right. On the right there are 2n from carbon dioxide, because each molecule has two oxygen atoms, plus n-3 from water, for a total of 3n-3. On the left there are the same number of oxygen atoms 3n-3, which means there are two times fewer molecules, because the molecule contains two atoms. That is (3n-3)/2 oxygen molecules.
Thus, we have compiled an equation for the combustion of benzene homologues in general form.